Access regulatory strategy consulting and In-Country Clinical Caretaker (ICCC) services that enable optimal product development of novel biopharmaceutical products in Japan.
Develop a Sound Regulatory Strategy in Japan
We invest in your submission’s success with our team of experienced regulatory leaders. Your submissions are backed by broad experience and specialized services in a wide range of therapeutic areas and indications.
- Access insights to navigate Japan’s evolving regulatory environment
- Achieve near-simultaneous global regulatory approval of your product
- Get advice on obtaining SAKIGAKE designation, expedited approval for regenerative medicines or orphan drug designation
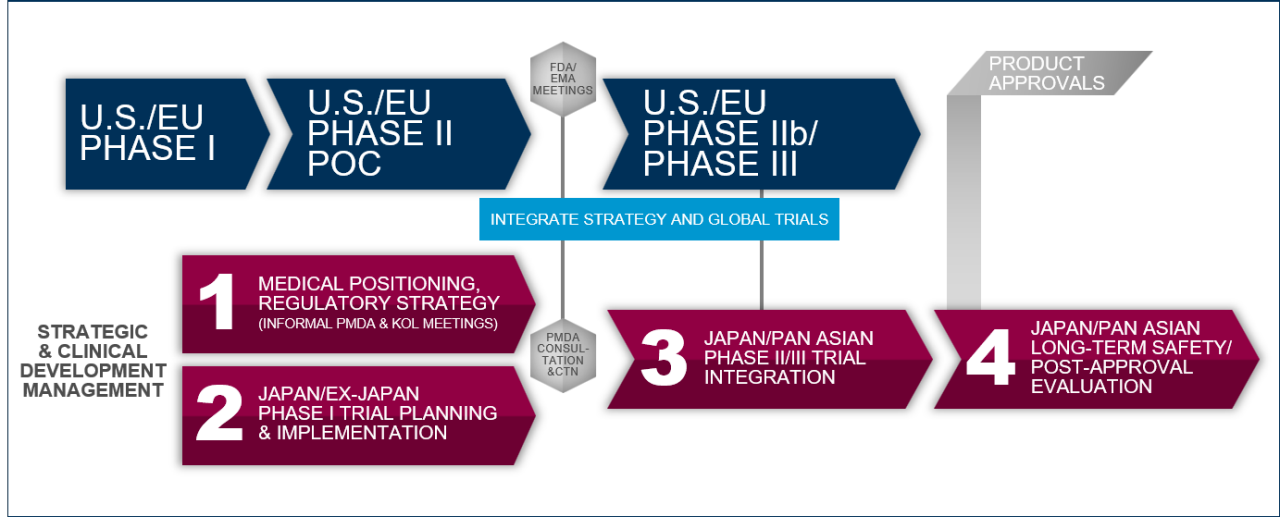
Enable the Optimal Development of Novel Biopharmaceutical Products for Japan
After decades of continuous harmonization of medical practices and with the successful initiative of the Ministry of Health, Labor and Welfare (MHLW) in Japan to create a regulatory framework aligned with the U.S. and EU, Japan has become well integrated into the global drug development landscape.
This steady regulatory reform that made Japan’s integration into U.S. and EU-led drug development programs possible, MHLW and Pharmaceuticals and Medical Devices Agency (PMDA) have now brought forth dynamic initiatives aimed at establishing Japan as a global leader in the development of breakthrough drugs addressing high, unmet medical needs.
Leveraging its multi-disciplinary team of drug development experts, we can not only successfully integrate Japan into global drug development programs but also work with clients to navigate these novel regulatory pathways such as SAKIGAKE, advance approval of regenerative medicines or support a product’s designation as an orphan drug.
Let us help you design development programs and increase your chances of success.