Access critical support for your end-to-end strategy's registration and commercialization.
Achieve your commercial goals and add value to your program – from the very start – with a committed partner.
A guide for navigating today’s complex environment
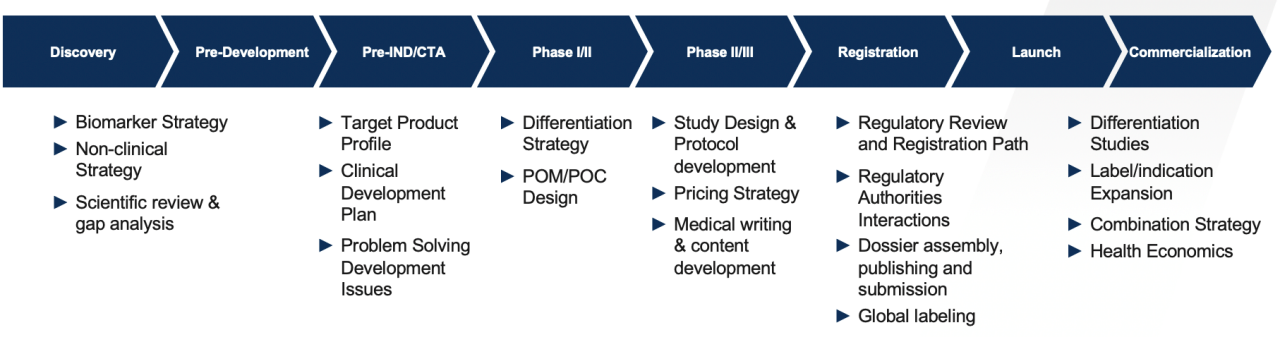
Reaching commercialization is a complex and iterative process. To be successful in the evolving drug development landscape, you must:
- Find the fastest path for reviewing your molecule
- Traverse an intricate development process
- Reduce inefficiencies at multiple stages
- Ensure commercial uptake
You need an experienced partner who knows how to help you reach your long-term commercial goal. Count on us to provide an innovative regulatory strategy – at any stage of your development.
An advocate, aligned with your resources
Whether you need strategic advice on your nonclinical strategy or registration and commercialization or advice on your next steps ahead, we can meet every aspect of your regulatory needs, regardless of your company size or product development stage.
Enhance your global strategy, agency meetings and interactions
Integrated global drug development has become the norm where biotech and pharmaceutical companies can now achieve near simultaneous regulatory approval of products worldwide. You need an experienced partner who can review your data, develop your submission and act as your guide through critical agency meetings. Whether you are following the traditional submission route or seeking fast-track or orphan drug designation, we have on-the-ground specialists who can work directly with your team and, if needed, even act on your behalf at agency meetings.