Pharmaceutical companies working in rare diseases rely on innovative partnerships to strategically develop their drugs in this challenging space. Count on our rare disease and pediatric team to provide critical expertise to advance your rare disease drug candidate to market.
Boost your rare disease patient enrollment
Patient access, recruitment and retention can be challenging in any trial but are uniquely difficult issues when working within rare diseases. Don’t let this hurdle stand in the way of developing your rare disease therapeutics. We utilize powerful analytical solutions such as Xcellerate® Clinical Trial Optimization® and access timely, real-world Labcorp diagnostic lab result data, global Central Labs investigator performance data and patient Intelligence input that enable us to deliver faster patient enrollment and enhanced patient retention. In addition to this data, our strong relationships with leading advocacy groups, fit-for-purpose tools to support and educate study teams, sites, patients and their families, and dedicated, passionate and experienced recruitment and retention team make us the right partner to bring your product to market faster.
Work with us to help improve your study’s visibility and credibility to create patient-centric solutions that reduce recruiting risks and maintain your program's momentum.
Leverage expertise and accelerate orphan drug development
Rare diseases are often poorly understood from a clinical perspective, highlighting the need for innovative study planning strategies. Our experts can help direct your rare disease clinical trial to yield more relevant results with an efficient study design that evaluates your desired outcomes and incorporates validated endpoints. Enable the development and collection of key clinical data in your studies with our innovative tools and approaches including biomarker discovery/development, mobile health, virtual natural histories and virtual trials.
With us as your partner, you’ll access a unique combination of dedicated rare disease therapeutic area expertise alongside comprehensive operational support to assist with every aspect of your global study. Together, we’ll develop robust study planning strategies that anticipate regulatory hurdles, navigate clinical complexities and accelerate your path forward.
Maximize your commercial potential
Even though orphan drugs address urgent unmet medical needs, understanding market impact is still crucial to maximizing your investment in an orphan indication. By evaluating payers, providers and patients, we can generate real-world, value-based evidence that helps you determine pricing and set your market access strategies.
Work with a team that combines medical, scientific, operational and economic expertise with extensive clinical trial experience to enable your rare disease clinical program and enhance your potential for a higher valuation and return on investment.
Supporting rare disease companies and overcoming orphan drug challenges
Developing an orphan drug to target a rare disease requires relevant experience in the therapeutic area and operational flexibility to handle a rare disease clinical trial’s unique challenges. We offer a wide variety of solutions—tailored for and dedicated to rare disease drug development—at every point from drug discovery to commercialization.
- Access more than 5000 assays, including relevant biomarkers, through our central labs and Labcorp specialty testing groups
- Leverage 120 genetic counselors through Labcorp and take advantage of genomic stratification for patient selection
- Trust in our proven logistical solutions to deliver >99% sample receipt within stability to maximize your sample yield
- Unlock your product’s market potential with more than 20 years of market access consulting experience for rare and orphan drugs in development
Whether you’re running a smaller Phase I study or delivering a global Phase II-III trial, you can rely on our global capabilities and operational expertise in rare disease studies to fully meet your drug program’s needs and deliver consistent results.
An experienced approach to advance your rare disease program
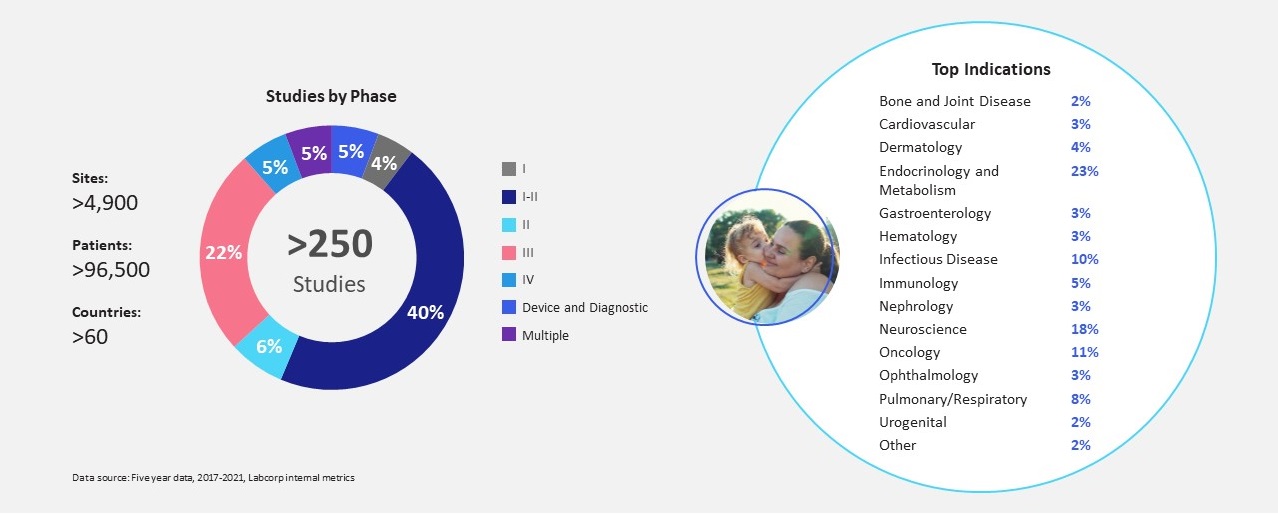
Beyond helping you secure rare disease volunteers to meet your recruitment needs, we also offer deep experience with developing orphan drugs, with a track record supporting more than 50 orphan indications and 103 rare disease studies across dozens of countries and thousands of sites. Pediatric-focused rare disease studies are even more complex, and our team has proven successful in the management of more than 25 such studies worldwide.
Let our multidisciplinary experts put their experience to work to advance your rare disease drug development program and transform obstacles into opportunities. Together, we’ll improve your potential for success and make a difference in urgent unmet medical needs.